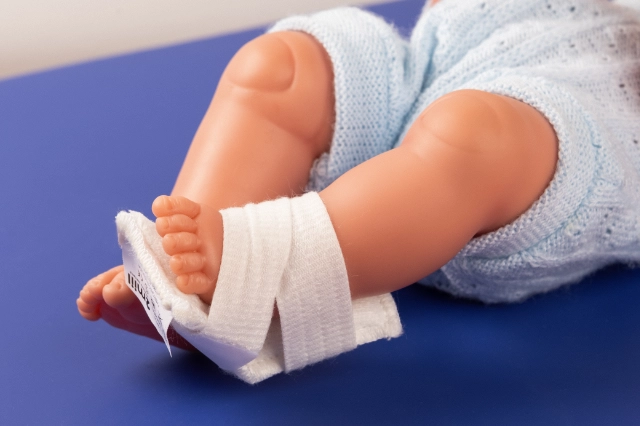
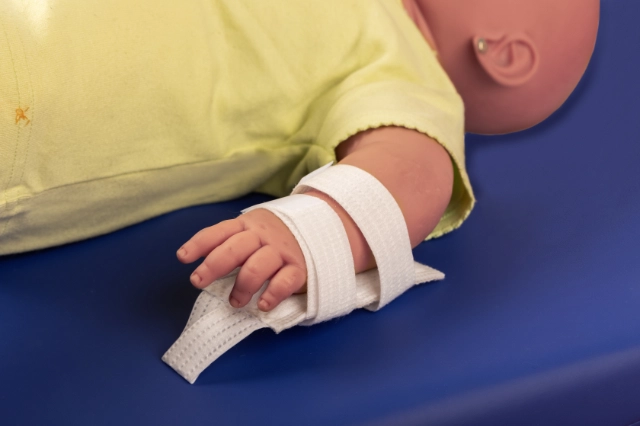
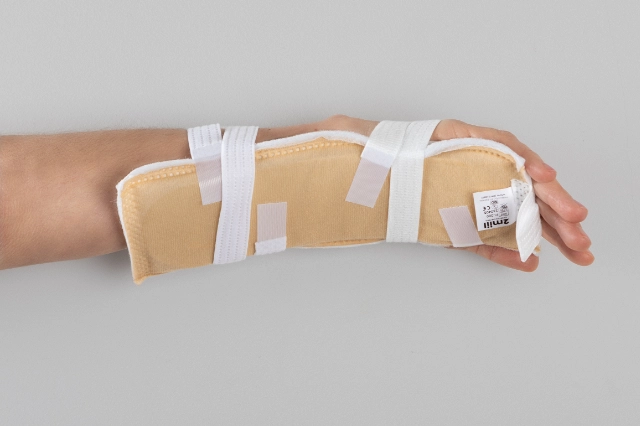
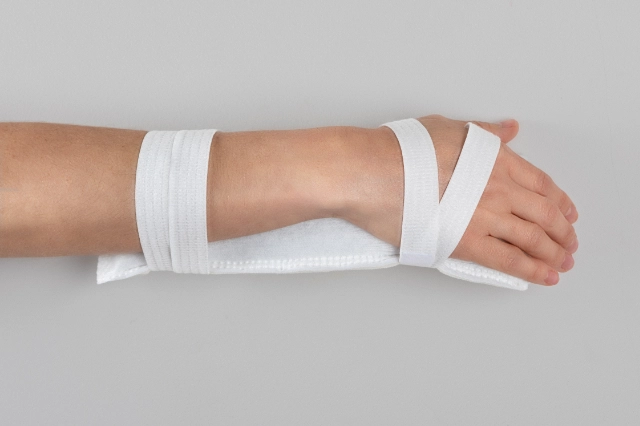
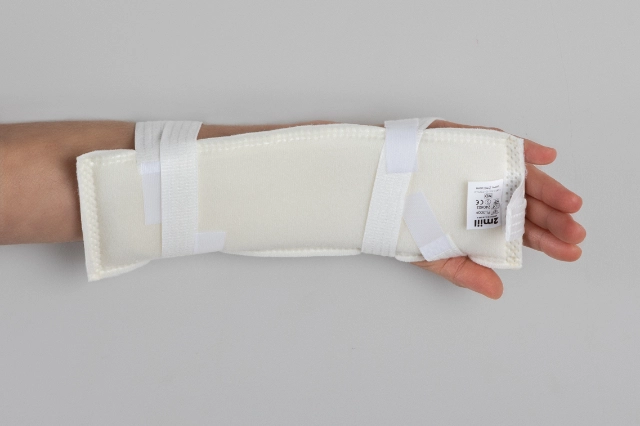
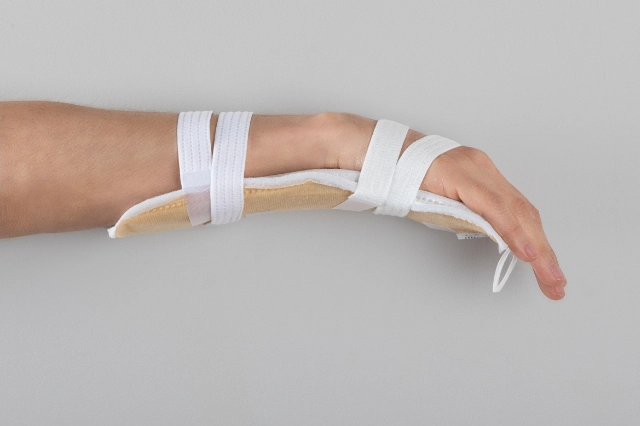
MOULDABLE SPLINTS FOR FIXATION OF I.V. CANNULAE.
2miil's mouldable I.V. cannula fixation splints are designed to provide secure and comfortable support for I.V. cannulae in paediatric and neonatal settings. These splints are manufactured from flexible, mouldable materials that allow for a precise fit, ensuring the stability of the cannulae without compromising patient comfort. They are ideal for use on newborns and infants, where gentleness and precision are essential. The ergonomic design of the splints makes them easy to apply and adjust, reducing the risk of accidental displacement and improving safety during intravenous treatment.
FI-300 / FI-305 / FI-310 / FI-311 / FI-312
Application
Application product for intra-arterial/intravenous postural fixation of patients, which have the characteristic of being mouldable for postural fixation.
Usage information
Mould the splint to fit the chosen position.
Fasten the straps with Velcro.
CUSHIONING CHARACTERISTICS
- FI-300 / FI-305 / FI-310 / FI-311 / FI-312
300 g/m2 resin-free polyester fibre padding 100 %, very soft and comfortable.
Full-surface cushioning for greater patient comfort.
- FI-300F / FI-305F / FI-310F / FI-311F / FI-312F
Polyester fibre padding 100 %, 300 gr/m2 skin contact, on a 4 mm thick, resin-free, very soft and comfortable polyether foam padding 100 % (PU), 4 mm thick.
Full-surface cushioning for greater patient comfort.
CHARACTERISTICS OF THE FABRICS
Both references are made of the same fabrics.
Inner skin contact layer of 45 gr/m2 polyester. Hypoallergenic.
Outer layer, polyamide 100 %, perfect adhesion to Velcro. Hypoallergenic.
Made of textiles certified according to Oeko-Tex Standard 100.
(Certification that guarantees that the fabrics are free of substances harmful to health, in addition to a higher quality of the product in terms of health and sustainability).
CHARACTERISTICS OF THE TAPES
Made with different fabrics sealed together by ultrasound, creating a micro-padding for comfort, adaptability and resistance. Soft and non-irritating to the skin.
INTERIOR COATING
Radiolucent, formable, radio-translucent aluminium sheet, wrapped in genuine 2miil cushioning.
Different sizes depending on the reference. Rounded edges.
Warnings and Recommendations
Using the product on a single patient could lead to cross-infection.
Do not use the product on open wounds or injured skin.
Do not use the product if it is damaged, dirty or contaminated.
Storage and shelf life:
- Store in a cool, dry place.
- Avoid exposure to direct sunlight.
- The shelf life is determined by the type and amount of use of the product in the same patient.
- Single use per patient.
- Shelf life: 5 years from date of manufacture.
LOGISTICS

FI-300 / FI-300F
SIZE: ADOLESCENT / ADULT
SIZE: 26 x 10 CM
UDS/BOX: 60
GTIN:
FI-300: 8435194403078
FI-300F: 8435194408240
(*) Tolerance: +/- 1 cm
(*) All references can be produced WITHOUT TAPE (REF. + _SC)
FI-305 / FI-305F
SIZE: SCHOOL / ADULT
SIZE: 20 x 10 CM
UDS/BOX: 60
GTIN:
FI-305: 8435194403115
FI-305F: 8435194408264
(*) Tolerance: +/- 1 cm
(*) All references can be produced WITHOUT TAPE (REF. + _SC)
FI-310 / FI-310F
SIZE: PEDIATRIC
SIZE: 16,5 x 6 CM*.
UDS/BOX: 40
GTIN:
FI-310: 8435194403153
FI-310F: 8435194408288
(*) Tolerance: +/- 1 cm
(*) All references can be produced WITHOUT TAPE (REF. + _SC)
FI-311 / FI-311F
SIZE: NEONATAL
SIZE: 10 x 5 CM
UDS/BOX: 80
GTIN:
FI-311: 8435194403191
FI-311F: 8435194408301
(*) Tolerance: +/- 1 cm
(*) All references can be produced WITHOUT TAPE (REF. + _SC)
FI-312 / FI-312F
SIZE: PREMATURE
SIZE: 9 x 4 CM* 9 x 4 CM* 9 x 4 CM* 9 x 4 CM* 9 x 4 CM* 9 x 4 CM
UDS/BOX: 100
GTIN:
FI-312: 8435194408516
FI-312F: 8435194408530
(*) Tolerance: +/- 1 cm
(*) All references can be produced WITHOUT TAPE (REF. + _SC)